Introduction. Olutasidenib is an FDA-approved, targeted therapy for IDH1-mutated (mIDH1) AML. Among 147 patients with relapsed/refractory mIDH1 AML, 51 (35%) achieved a complete response (CR) or CR with partial hematologic recovery (CRh) after treatment with olutasidenib, with a duration of response of 25.9 months. We conducted sub-analyses to better understand which factors might be predictive of response to therapy.
Methods. Patients were divided into groups based on achieving an overall response (CR, CRh, CRi, PR, MLFS) or having best response of stable disease or progression. Groups were compared to determine if there were baseline patient or disease characteristics that might predict attainment of an overall response to olutasidenib. The study was not powered to statistically evaluate subpopulations. Descriptive statistics are provided, and differences of 20% or more between groups were noted. Data cutoff date is June 2021.
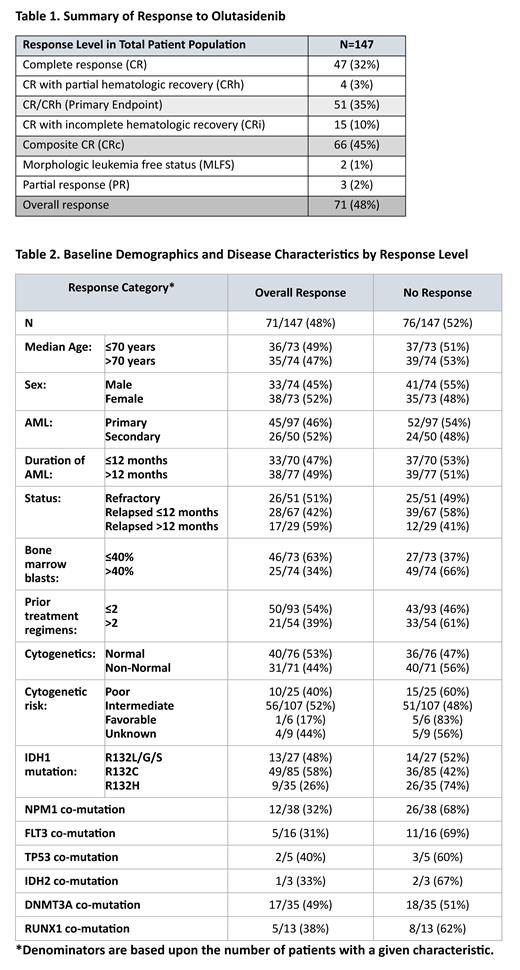
Results. As of data cutoff, 71 (48%) patients had achieved an overall response; the remaining 76 patients had stable disease (SD), clinical benefit (CB), disease progression (PD) or were not evaluated (due to short time on trial). Composite CR (CR, CRh, CRi) was achieved in 66/71 (93%) of overall responders, with PR and MLFS comprising only 3% of overall responders ( Table 1). With a median follow-up of 10.2 months (range 0.2-38.1), 20 responders were continuing treatment with ongoing responses at the time of data cutoff while 51 had discontinued; reasons for discontinuation were HSCT (n=14 all of whom had ongoing responses), disease progression (n=24), adverse event (n=8), physician decision (n=4) and protocol deviation (1).
Responses to olutasidenib therapy were observed across a broad range of patient demographics and disease characteristics, most of which did not appear to impact the rate of response to olutasidenib ( Table 2). Bone marrow blast count at baseline correlated with response, with 63% of patients with baseline counts ≤40% achieving an overall response compared with 34% in those with baseline counts >40%. More patients with R132C (58%) and R132L/G/S (48%) mutations achieved an overall response compared to those with an R132H mutation (26%) However, a previous analysis showed that patients with the R132H mutation were more likely to have a FLT3 and/or NPM1 mutation, which are associated with a poor prognosis (de Botton S, et al, Blood 2021). Although fewer patients in the overall responder groups had FLT3 or NPM1 mutations compared with the non-responder group, 12/38 (32%) with NPM1 and 5/16 (31%) with FLT3 had an overall response. In addition, 2/5 (40%) patients with the TP53 co-mutation had an overall response to olutasidenib.
While group outcomes for specific characteristics of disease may suggest a worse prognosis, individual patients treated with olutasidenib may achieve an overall response despite the impact of these negative factors. Two patients in this analysis had mIDH1-R132H mutations as well as FLT3 and NPM1 co-mutations and primary relapsed AML. One achieved a CRh and proceeded to transplant after a DOR of 15.5 months, and the other achieved a CRi lasting 1.3 months and then progressed. The baseline bone blast count was 53% in the first patient and 80% in the second. Two of the responders in this analysis had mIDH1-R132C and a TP53 co-mutation with refractory AML; one achieved a CR and proceeded to transplant after a DOR of 6 months, and the other had a PR and progressed after 3 months. The baseline bone blast count was 25% in the first patient and 65% in the second patient.
Conclusions. This post-hoc analysis demonstrated that response to olutasidenib occurred across a wide variety of patient types and disease characteristics, including those generally associated with a poor prognosis. Baseline bone blast count was strongly predictive of response to treatment. Further evaluation of these observations is needed.
Disclosures
Wang:Abbvie: Consultancy; Astellas: Consultancy, Speakers Bureau; BMS: Consultancy; Gilead: Consultancy; GlaxoSmithKline: Consultancy; Jazz: Consultancy; Kite: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; PharmaEssentia: Consultancy; Takeda: Consultancy; Dava oncology: Speakers Bureau; Kura Oncology: Speakers Bureau. Jonas:Loxo: Research Funding; Incyte: Research Funding; Immune-Onc: Research Funding; Hanmi: Research Funding; Genentech/Roche: Research Funding; Forty-Seven: Research Funding; Forma: Research Funding; F. Hoffmann-La Roche: Research Funding; Celgene: Research Funding; Aptose: Research Funding; AROG: Research Funding; Amgen: Research Funding; Takeda: Consultancy; Servier: Consultancy; Rigel: Consultancy, Other: travel reimbursement ; Pfizer: Consultancy, Research Funding; Kymera: Consultancy; Jazz: Consultancy, Research Funding; GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; Gilead: Consultancy, Other: data monitoring committee , Research Funding; Genentech: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; BMS: Consultancy, Research Funding; AbbVie: Consultancy, Other: travel reimbursement , Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding; Treadwell: Research Funding. Watts:Takeda: Consultancy, Research Funding; Immune Systems Key: Research Funding; Rafael Pharma: Consultancy; Reven Pharma: Consultancy; Servier: Consultancy; Rigel: Consultancy; BMS: Consultancy; Aptose: Consultancy; Daiichi Sankyo: Consultancy. Jurcic:Bristol Myers Squibb: Consultancy, Research Funding; Seattle Genetics: Research Funding; Syros Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Ionis Pharmaceuticals: Research Funding; Forma Therapeutics: Research Funding; Sumitomo Pharma: Research Funding; Rigel Pharmaceuticals: Consultancy; Gilead/Forty Seven: Research Funding; Blueprint Medicines: Research Funding; AbbVie: Research Funding. Ferrell:Novartis: Research Funding. Dinner:Kite/Gilead: Research Funding; Novartis: Research Funding; BMS: Research Funding; Rigel: Research Funding; Pfizer: Research Funding. Seiter:Blueprint: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Servier: Speakers Bureau; Alexion: Honoraria; Incyte: Speakers Bureau; CTI: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Cortes:Abbvie: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Gilead: Consultancy; Takeda: Consultancy, Honoraria; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma Therapuetic: Consultancy; Pfizer: Consultancy, Research Funding.